Introduction to CryoET¶
What is CryoET?¶
Cryo-electron tomography (CryoET) is a technique that provides researchers with a 3D view of cells, subcellular components (e.g., organelles), viruses, and proteins. Detailed 3D image reconstructions generated through CryoET enable researchers to answer questions that are critical for understanding cellular function and response, such as:
How are subcellular components and proteins spatially organized within a cell?
How do subcellular components and proteins interact with each other?
How do cells change in response to stress, injury, or disease?
How do infectious agents interact with host cells?
What makes CryoET special?¶
Thanks to CryoET, researchers can now bridge the gap between techniques that enable them to study cells at the micron scale (e.g., light microscopy) and molecular structures at the atomic-level (e.g., X-ray crystallography). Although there are other techniques that generate 3D models of subcellular components, viruses, and proteins, these techniques require isolation of single particles. CryoET is the only technique that enables researchers to study biological samples in their native environment at near atomic resolution while providing context about their location, conformation, and interactions within a cell or medium. It is a powerful technique that can provide structural information at a range of resolutions, from whole cells to molecules, including the atomic structure of particles with resolutions near 3 angstrom (Å; 1 Å = 0.1 nm).
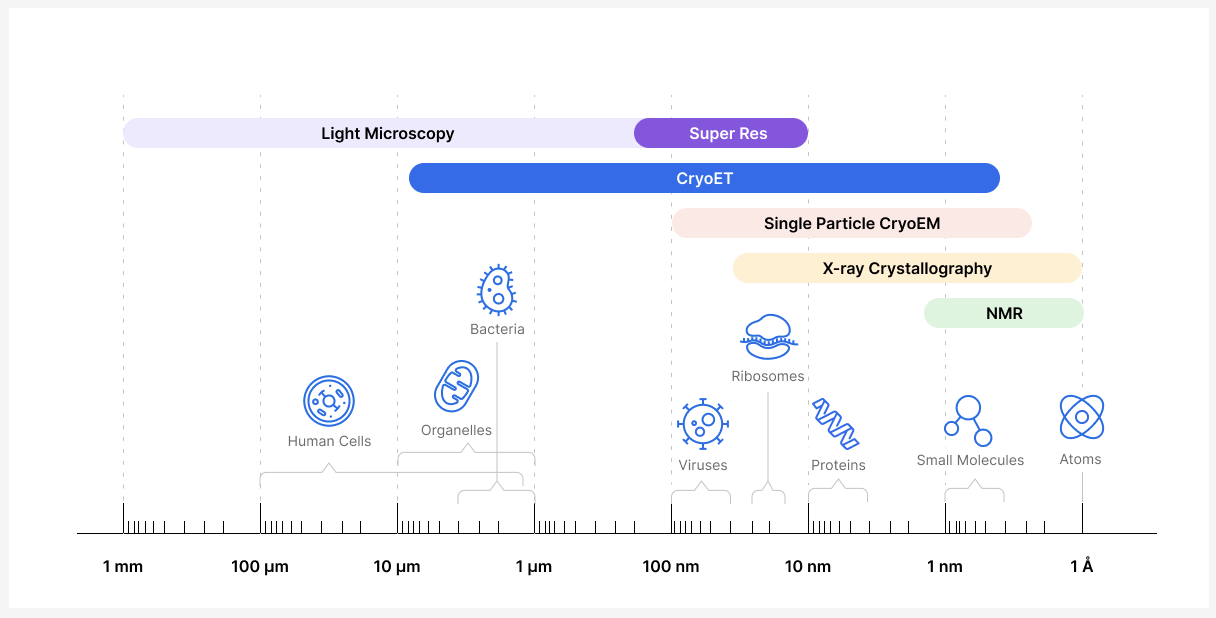
What is the technology behind CryoET?¶
The main technologies that make CryoET possible are highlighted in its name, including: cryogenic techniques, electron microscopy and tomography.
Cryo¶
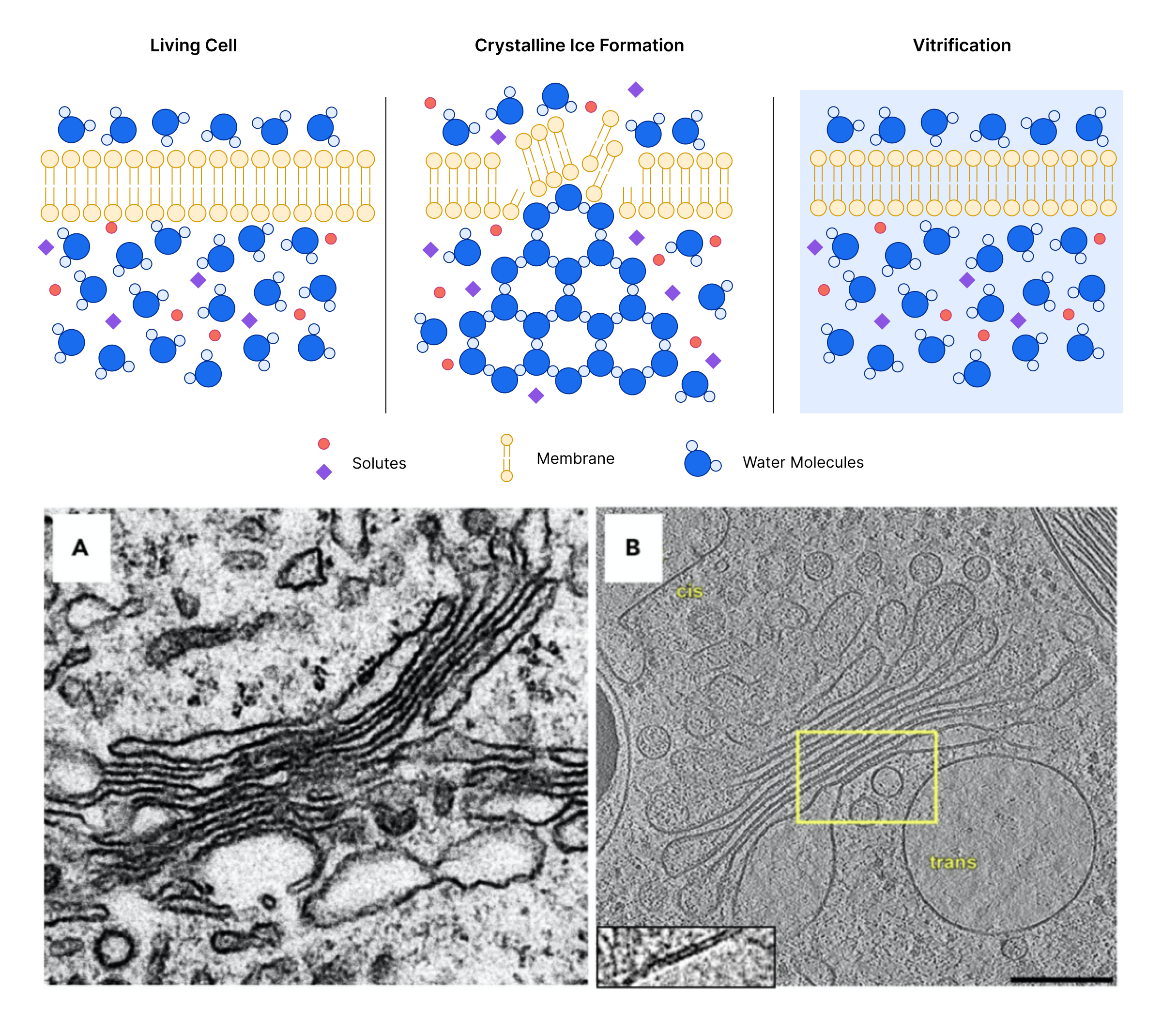
Cryo refers to cryogenic freezing techniques that are used to fix samples without the use of chemical fixatives or stains. Cryo-fixation happens so quickly that biological material and processes are frozen in their hydrated, near native state before ice crystals start to form. This flash-freezing process that preserves the natural structure of the sample in a glass-like state is known as vitrification because it embeds the sample in amorphous (vitreous) ice. One of the first steps during CryoET sample preparation is to “vitrify” samples. Cryo-fixation also protects the sample when exposed to the high-vacuum environment of the electron microscope.
Electron¶
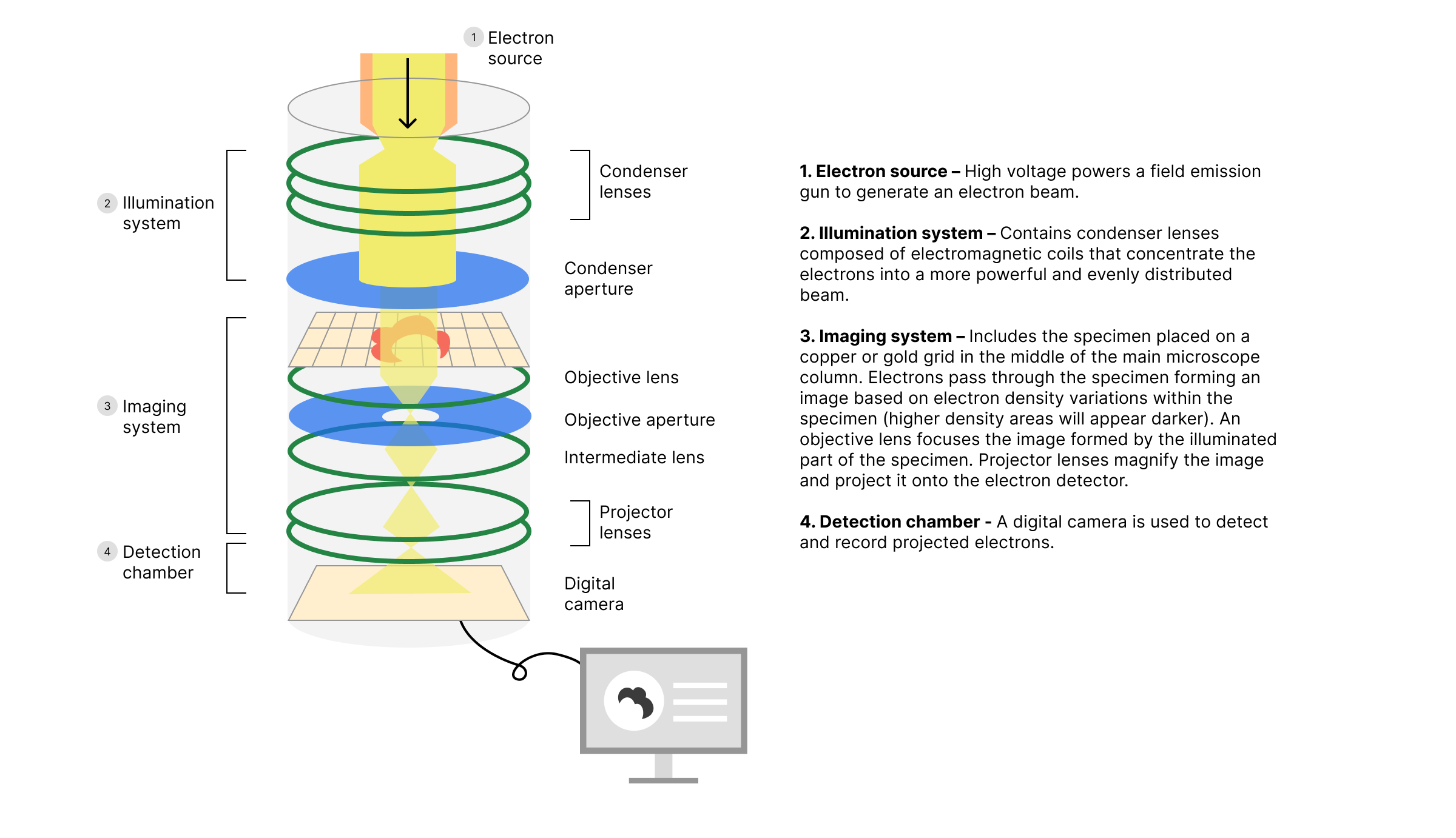
Electron specifies that CryoET is an electron microscopy technique where an electron beam interacts with the sample to project an image. Electron microscopy is used to view and gain structural information about subcellular and viral components. Electron wavelengths are small enough to interact with these components and produce images based on those interactions. CryoET falls under the transmission electron microscopy category, where electrons pass through the sample and illuminate film or a digital camera. High electron density components cast stronger shadows than lighter density ones, thus producing a 2D projection of the material in the sample. Click here for a video explaining how electron microscopes work.
Tomography¶
Tomography refers to an imaging technique that provides 3D information of an object by capturing projection images from multiple angles. CryoET collects 2D images representing rotational views or tilted projections from a sample. The collected 2D images, known as a tilt series, are then transformed into volume providing spatial information. Reconstructed 3D images are known as tomograms.
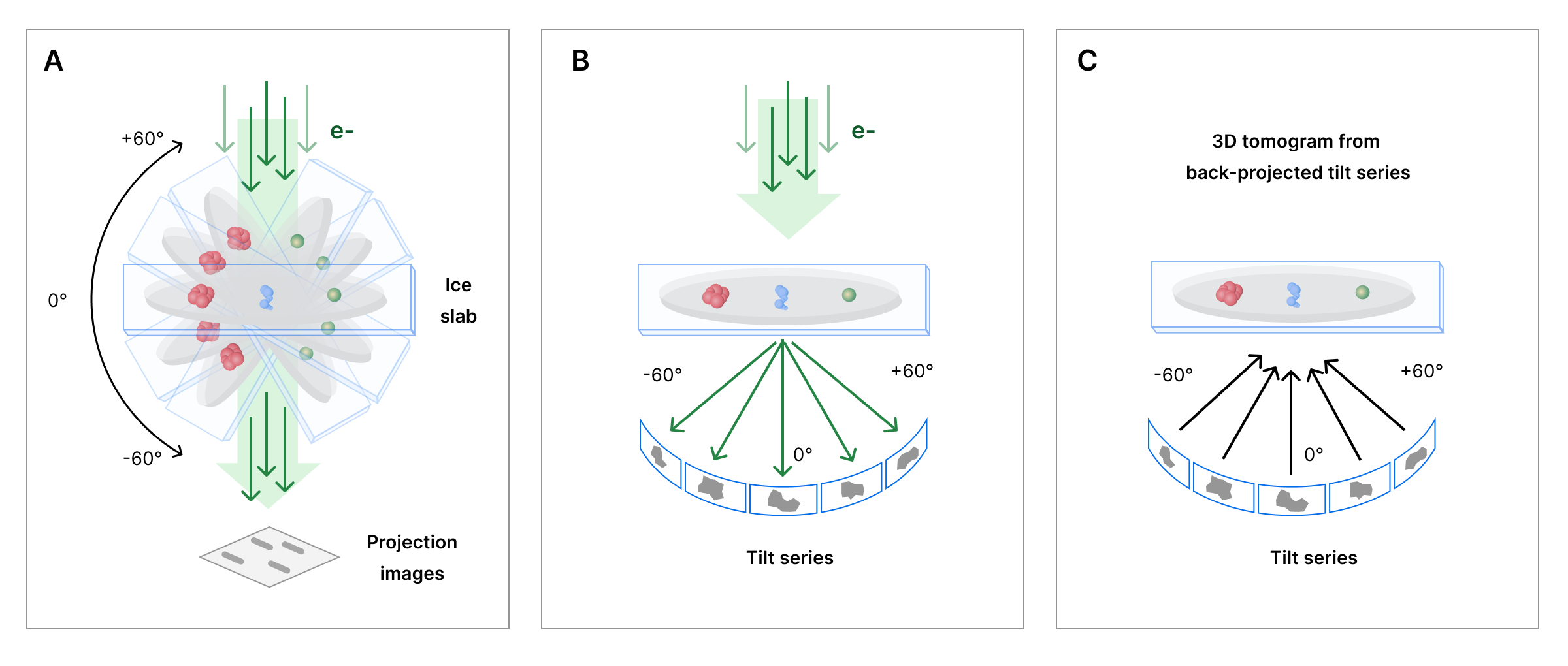
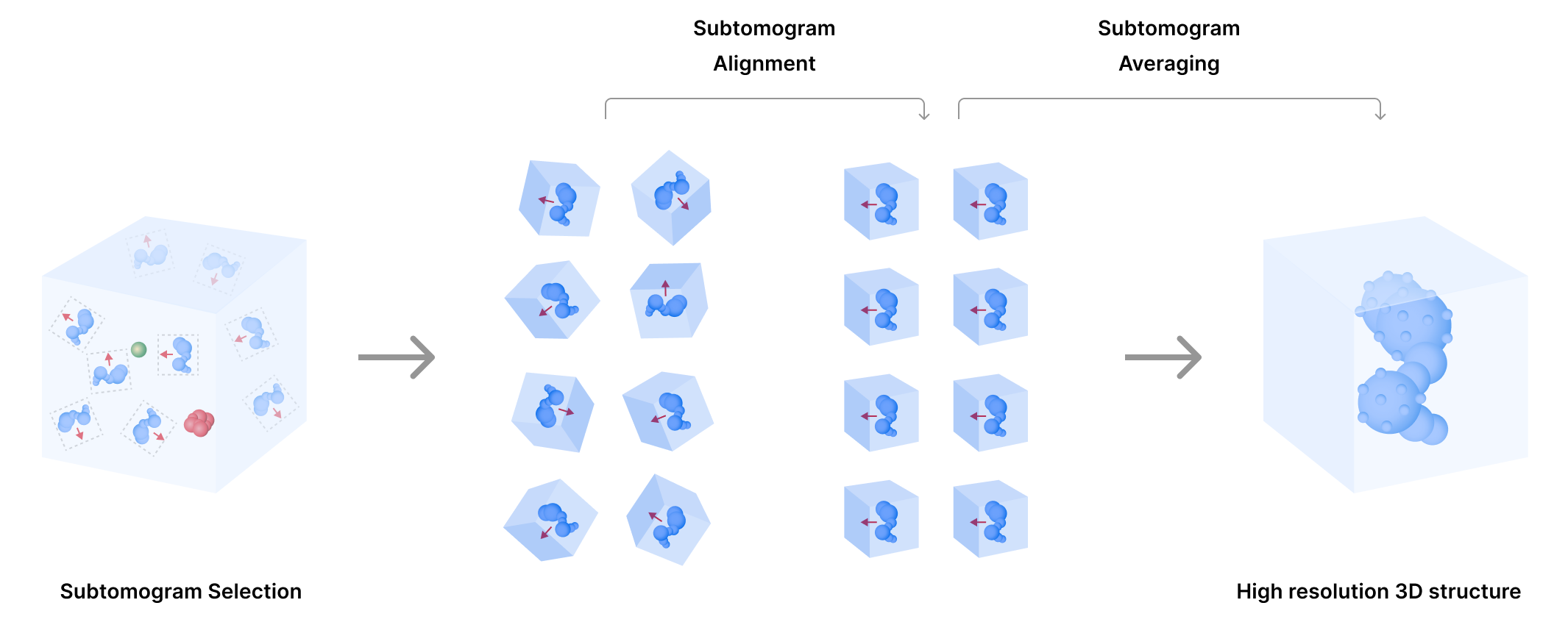
Computational efforts are continuously optimizing tomogram reconstruction (e.g., automation of image pre-processing steps, such as image alignments, and improving signal-to-noise ratios). High quality tomograms can then be used to computationally improve the resolution of smaller, repetitive particles within tomograms to reconstruct their structure. This single particle reconstruction from tomograms is known as subtomogram averaging. Through subtomogram averaging, CryoET data can lead to molecular structures with resolutions near 3 Å.