Exploring all data from a tissue
This tutorial provides a series of examples for how to explore and query the Census in the context of a single tissue, lung. We will summarize cell and gene metadata, then fetch the single-cell expression counts and perform some basic data explorations via Scanpy
Contents
Learning about the human lung data.
Learning about cells of the lung.
Learning about genes of the lung .
Fetching all single-cell human lung data from the Census.
Calculating QC metrics of the lung data.
Creating a normalized expression layer and embeddings.
⚠️ Note that the Census RNA data includes duplicate cells present across multiple datasets. Duplicate cells can be filtered in or out using the cell metadata variable is_primary_data which is described in the Census schema.
Learning about the lung data in the Census
First we will open the Census. If you are not familiar with the basics of the Census API you should take a look at notebook Learning about the CZ CELLxGENE Census
[1]:
import cellxgene_census
import numpy as np
import pandas as pd
import scanpy as sc
census = cellxgene_census.open_soma()
The "stable" release is currently 2023-07-25. Specify 'census_version="2023-07-25"' in future calls to open_soma() to ensure data consistency.
Let’s first take a look at the number of cells from human lung:
[2]:
summary_table = census["census_info"]["summary_cell_counts"].read().concat().to_pandas()
summary_table.query("organism == 'Homo sapiens' & category == 'tissue_general' & label =='lung'")
[2]:
| soma_joinid | organism | category | ontology_term_id | unique_cell_count | total_cell_count | label | |
|---|---|---|---|---|---|---|---|
| 980 | 980 | Homo sapiens | tissue_general | UBERON:0002048 | 2907156 | 6011592 | lung |
There you can see the total of cells of under total_cell_count and the unique number cells under unique_cell_count (i.e. after removing cells that were included in multiple datasets).
Let’s now take a look at the cell and gene information of this slice of the Census.
Learning about cells of lung data
Let’s load the cell metadata for all lung cells and select only the unique cells using is_primary_data.
[3]:
lung_obs = cellxgene_census.get_obs(
census, "homo_sapiens", value_filter="tissue_general == 'lung' and is_primary_data == True"
)
lung_obs
[3]:
| soma_joinid | dataset_id | assay | assay_ontology_term_id | cell_type | cell_type_ontology_term_id | development_stage | development_stage_ontology_term_id | disease | disease_ontology_term_id | ... | is_primary_data | self_reported_ethnicity | self_reported_ethnicity_ontology_term_id | sex | sex_ontology_term_id | suspension_type | tissue | tissue_ontology_term_id | tissue_general | tissue_general_ontology_term_id | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 5945423 | 9f222629-9e39-47d0-b83f-e08d610c7479 | 10x 3' v2 | EFO:0009899 | native cell | CL:0000003 | unknown | unknown | normal | PATO:0000461 | ... | True | unknown | unknown | unknown | unknown | cell | lung | UBERON:0002048 | lung | UBERON:0002048 |
| 1 | 5945426 | 9f222629-9e39-47d0-b83f-e08d610c7479 | Drop-seq | EFO:0008722 | ciliated columnar cell of tracheobronchial tree | CL:0002145 | 57-year-old human stage | HsapDv:0000151 | pulmonary fibrosis | MONDO:0002771 | ... | True | unknown | unknown | male | PATO:0000384 | cell | lung | UBERON:0002048 | lung | UBERON:0002048 |
| 2 | 5945428 | 9f222629-9e39-47d0-b83f-e08d610c7479 | 10x 3' transcription profiling | EFO:0030003 | CD8-positive, alpha-beta T cell | CL:0000625 | unknown | unknown | squamous cell lung carcinoma | MONDO:0005097 | ... | True | unknown | unknown | unknown | unknown | cell | lung | UBERON:0002048 | lung | UBERON:0002048 |
| 3 | 5945432 | 9f222629-9e39-47d0-b83f-e08d610c7479 | 10x 3' v2 | EFO:0009899 | CD4-positive, alpha-beta T cell | CL:0000624 | unknown | unknown | lung adenocarcinoma | MONDO:0005061 | ... | True | unknown | unknown | unknown | unknown | cell | lung | UBERON:0002048 | lung | UBERON:0002048 |
| 4 | 5945441 | 9f222629-9e39-47d0-b83f-e08d610c7479 | 10x 3' v2 | EFO:0009899 | CD8-positive, alpha-beta T cell | CL:0000625 | unknown | unknown | lung adenocarcinoma | MONDO:0005061 | ... | True | unknown | unknown | unknown | unknown | cell | lung | UBERON:0002048 | lung | UBERON:0002048 |
| ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... |
| 2907151 | 56400868 | 8c42cfd0-0b0a-46d5-910c-fc833d83c45e | 10x 3' v2 | EFO:0009899 | pericyte | CL:0000669 | 51-year-old human stage | HsapDv:0000145 | normal | PATO:0000461 | ... | True | unknown | unknown | female | PATO:0000383 | cell | lung | UBERON:0002048 | lung | UBERON:0002048 |
| 2907152 | 56400869 | 8c42cfd0-0b0a-46d5-910c-fc833d83c45e | 10x 3' v2 | EFO:0009899 | pericyte | CL:0000669 | 51-year-old human stage | HsapDv:0000145 | normal | PATO:0000461 | ... | True | unknown | unknown | female | PATO:0000383 | cell | lung | UBERON:0002048 | lung | UBERON:0002048 |
| 2907153 | 56400870 | 8c42cfd0-0b0a-46d5-910c-fc833d83c45e | 10x 3' v2 | EFO:0009899 | pericyte | CL:0000669 | 51-year-old human stage | HsapDv:0000145 | normal | PATO:0000461 | ... | True | unknown | unknown | female | PATO:0000383 | cell | lung | UBERON:0002048 | lung | UBERON:0002048 |
| 2907154 | 56400871 | 8c42cfd0-0b0a-46d5-910c-fc833d83c45e | 10x 3' v2 | EFO:0009899 | pericyte | CL:0000669 | 51-year-old human stage | HsapDv:0000145 | normal | PATO:0000461 | ... | True | unknown | unknown | female | PATO:0000383 | cell | lung | UBERON:0002048 | lung | UBERON:0002048 |
| 2907155 | 56400872 | 8c42cfd0-0b0a-46d5-910c-fc833d83c45e | 10x 3' v2 | EFO:0009899 | pericyte | CL:0000669 | 51-year-old human stage | HsapDv:0000145 | normal | PATO:0000461 | ... | True | unknown | unknown | female | PATO:0000383 | cell | lung | UBERON:0002048 | lung | UBERON:0002048 |
2907156 rows × 21 columns
You can see that the number or rows represents the total number of unique lung cells in the Census. Now let’s take a deeper dive into the characteristics of these cells.
Datasets
First let’s start by looking at what are the datasets and collections from CELLxGENE Discover contributing to lung. For this we will use the dataset table at census["census-info"]["datasets"] that contains metadata of all datasets used to build this Census.
[4]:
census_datasets = (
census["census_info"]["datasets"]
.read(column_names=["collection_name", "dataset_title", "dataset_id", "soma_joinid"])
.concat()
.to_pandas()
)
census_datasets = census_datasets.set_index("dataset_id")
census_datasets
[4]:
| collection_name | dataset_title | soma_joinid | |
|---|---|---|---|
| dataset_id | |||
| f171db61-e57e-4535-a06a-35d8b6ef8f2b | Spatial multiomics map of trophoblast developm... | donor_p13_trophoblasts | 0 |
| ecf2e08e-2032-4a9e-b466-b65b395f4a02 | Spatial multiomics map of trophoblast developm... | All donors trophoblasts | 1 |
| 74cff64f-9da9-4b2a-9b3b-8a04a1598040 | Spatial multiomics map of trophoblast developm... | All donors all cell states (in vivo) | 2 |
| 5af90777-6760-4003-9dba-8f945fec6fdf | Mapping single-cell transcriptomes in the intr... | Single-cell transcriptomic datasets of Renal c... | 3 |
| bd65a70f-b274-4133-b9dd-0d1431b6af34 | Single-cell sequencing links multiregional imm... | Single-cell sequencing links multiregional imm... | 4 |
| ... | ... | ... | ... |
| f9ad5649-f372-43e1-a3a8-423383e5a8a2 | Molecular characterization of selectively vuln... | Molecular characterization of selectively vuln... | 588 |
| 456e8b9b-f872-488b-871d-94534090a865 | Single-cell atlas of peripheral immune respons... | Single-cell atlas of peripheral immune respons... | 589 |
| 2adb1f8a-a6b1-4909-8ee8-484814e2d4bf | Construction of a human cell landscape at sing... | Construction of a human cell landscape at sing... | 590 |
| e04daea4-4412-45b5-989e-76a9be070a89 | A molecular cell atlas of the human lung from ... | Krasnow Lab Human Lung Cell Atlas, Smart-seq2 | 591 |
| 8c42cfd0-0b0a-46d5-910c-fc833d83c45e | A molecular cell atlas of the human lung from ... | Krasnow Lab Human Lung Cell Atlas, 10X | 592 |
593 rows × 3 columns
The obs cell metadata pandas.DataFrame contains a column dataset_id that can be used for joining to the census_dataset pandas.DataFrame we just created.
So let’s take a look at the cell counts per dataset_id of the lung slice and then join to the dataset table to append the human-readable labels.
[5]:
dataset_cell_counts = pd.DataFrame(lung_obs[["dataset_id"]].value_counts())
dataset_cell_counts = dataset_cell_counts.rename(columns={0: "cell_counts"})
dataset_cell_counts = dataset_cell_counts.merge(census_datasets, on="dataset_id")
dataset_cell_counts
[5]:
| count | collection_name | dataset_title | soma_joinid | |
|---|---|---|---|---|
| dataset_id | ||||
| 1e6a6ef9-7ec9-4c90-bbfb-2ad3c3165fd1 | 1028006 | High-resolution single-cell atlas reveals dive... | The single-cell lung cancer atlas (LuCA) -- ex... | 314 |
| 9f222629-9e39-47d0-b83f-e08d610c7479 | 784630 | The integrated Human Lung Cell Atlas | An integrated cell atlas of the human lung in ... | 56 |
| f7c1c579-2dc0-47e2-ba19-8165c5a0e353 | 217738 | A human cell atlas of fetal gene expression | Survey of human embryonic development | 483 |
| d8da613f-e681-4c69-b463-e94f5e66847f | 116313 | A molecular single-cell lung atlas of lethal C... | A molecular single-cell lung atlas of lethal C... | 80 |
| 576f193c-75d0-4a11-bd25-8676587e6dc2 | 90384 | HTAN MSK - Single cell profiling reveals novel... | Combined samples | 377 |
| d41f45c1-1b7b-4573-a998-ac5c5acb1647 | 82991 | HTAN MSK - Transcriptional connectivity of reg... | Transcriptional connectivity of regulatory T c... | 58 |
| 3dc61ca1-ce40-46b6-8337-f27260fd9a03 | 71752 | A human fetal lung cell atlas uncovers proxima... | All cells | 325 |
| 8c42cfd0-0b0a-46d5-910c-fc833d83c45e | 60993 | A molecular cell atlas of the human lung from ... | Krasnow Lab Human Lung Cell Atlas, 10X | 592 |
| 2672b679-8048-4f5e-9786-f1b196ccfd08 | 57019 | scRNA-seq assessment of the human lung, spleen... | Lung Parenchyma | 416 |
| 9dbab10c-118d-496b-966a-67f1763a6b7d | 49014 | COVID-19 immune features revealed by a large-s... | Large-scale single-cell analysis reveals criti... | 482 |
| 9968be68-ab65-4a38-9e1a-c9b6abece194 | 47909 | Charting human development using a multi-endod... | Developing Human Atlas | 78 |
| 3de0ad6d-4378-4f62-b37b-ec0b75a50d94 | 46500 | LungMAP — Human data from a broad age healthy ... | Single-cell multiomic profiling of human lungs... | 456 |
| 2f132ec9-24b5-422f-9be0-ccef03b4fe28 | 39778 | SARS-CoV-2 receptor ACE2 and TMPRSS2 are prima... | Lung | 312 |
| 1e5bd3b8-6a0e-4959-8d69-cafed30fe814 | 35699 | Emphysema Cell Atlas | immune cells | 130 |
| 53d208b0-2cfd-4366-9866-c3c6114081bc | 35682 | Tabula Sapiens | Tabula Sapiens - All Cells | 475 |
| 1b9d8702-5af8-4142-85ed-020eb06ec4f6 | 35419 | Cross-tissue immune cell analysis reveals tiss... | Global | 411 |
| 4ed927e9-c099-49af-b8ce-a2652d069333 | 35284 | Single-nucleus cross-tissue molecular referenc... | Single-nucleus cross-tissue molecular referenc... | 367 |
| 2adb1f8a-a6b1-4909-8ee8-484814e2d4bf | 33698 | Construction of a human cell landscape at sing... | Construction of a human cell landscape at sing... | 590 |
| 4b6af54a-4a21-46e0-bc8d-673c0561a836 | 18386 | Emphysema Cell Atlas | non-immune cells | 128 |
| 01209dce-3575-4bed-b1df-129f57fbc031 | 11059 | Single-cell transcriptomics of human T cells r... | Single-cell transcriptomics of human T cells r... | 531 |
| e04daea4-4412-45b5-989e-76a9be070a89 | 8657 | A molecular cell atlas of the human lung from ... | Krasnow Lab Human Lung Cell Atlas, Smart-seq2 | 591 |
| f9846bb4-784d-4582-92c1-3f279e4c6f0c | 176 | A human fetal lung cell atlas uncovers proxima... | Fibroblast and smooth muscle | 317 |
| f64e1be1-de15-4d27-8da4-82225cd4c035 | 55 | HTAN MSK - Single cell profiling reveals novel... | Immune cells | 370 |
| 810ac45f-8969-4698-b42c-652f802f75c2 | 10 | A human fetal lung cell atlas uncovers proxima... | Endothelium | 320 |
| 0ba16f4b-cb87-4fa3-9363-19fc51eec6e7 | 4 | A human fetal lung cell atlas uncovers proxima... | Myeloid | 326 |
These are all the datasets lung cells whose counts are reprensented in the column cell_counts. The top collections with lung data are:
Assays
Let’s use similar logic to take a look at all the assays available for human lung data. This tells us that most assays are from 10x technologies and sci-RNA-seq.
[6]:
lung_obs[["assay"]].value_counts()
[6]:
assay
10x 3' v2 1236968
10x 3' v3 702074
10x 5' v1 262323
sci-RNA-seq 217738
BD Rhapsody Whole Transcriptome Analysis 122902
10x 3' transcription profiling 97432
Drop-seq 65220
single cell library construction 58981
10x 5' v2 41852
microwell-seq 33698
Smart-seq2 25662
inDrop 25652
10x 3' v1 8638
Seq-Well 8016
Name: count, dtype: int64
Disease
And now let’s take a look at diseased cell counts, with normal indicating non-diseased cells.
[7]:
lung_obs[["disease"]].value_counts()
[7]:
disease
normal 1164084
lung adenocarcinoma 772120
COVID-19 331019
squamous cell lung carcinoma 209675
non-small cell lung carcinoma 120796
chronic obstructive pulmonary disease 55254
pulmonary fibrosis 51343
interstitial lung disease 45714
pneumonia 31923
pulmonary emphysema 31792
small cell lung carcinoma 31540
lung large cell carcinoma 21167
cystic fibrosis 17590
lymphangioleiomyomatosis 12374
pleomorphic carcinoma 10765
Name: count, dtype: int64
Sex
There doesn’t seem to be strong biases for sex.
[8]:
lung_obs[["sex"]].value_counts()
[8]:
sex
male 1402565
female 1122990
unknown 381601
Name: count, dtype: int64
Cell vs nucleus
The majority of data are from cells and not nucleus.
[9]:
lung_obs[["suspension_type"]].value_counts()
[9]:
suspension_type
cell 2468587
nucleus 438569
Name: count, dtype: int64
Cell types
Let’s take a look at the counts of the top 20 cell types.
[10]:
lung_obs[["cell_type"]].value_counts().head(20)
[10]:
cell_type
alveolar macrophage 291507
native cell 263362
CD4-positive, alpha-beta T cell 211456
CD8-positive, alpha-beta T cell 189471
macrophage 154415
type II pneumocyte 128463
epithelial cell of lower respiratory tract 105090
classical monocyte 102303
natural killer cell 95953
T cell 92846
stromal cell 87714
B cell 81125
malignant cell 75917
plasma cell 64551
epithelial cell 59353
fibroblast 45305
capillary endothelial cell 39416
regulatory T cell 36381
ciliated columnar cell of tracheobronchial tree 36049
epithelial cell of lung 35467
Name: count, dtype: int64
Sub-tissues
We can look at the original tissue annotations that were mapped to “lung”.
[11]:
lung_obs[["tissue"]].value_counts()
[11]:
tissue
lung 2576327
lung parenchyma 147410
alveolus of lung 54085
lingula of left lung 35284
upper lobe of right lung 32099
lower lobe of left lung 17854
right lung 12880
upper lobe of left lung 10113
left lung 9276
lower lobe of right lung 7981
middle lobe of right lung 3847
Name: count, dtype: int64
Learning about genes of lung data
Let’s load the gene metadata of the Census.
[12]:
lung_var = cellxgene_census.get_var(census, "homo_sapiens")
lung_var
[12]:
| soma_joinid | feature_id | feature_name | feature_length | |
|---|---|---|---|---|
| 0 | 0 | ENSG00000121410 | A1BG | 3999 |
| 1 | 1 | ENSG00000268895 | A1BG-AS1 | 3374 |
| 2 | 2 | ENSG00000148584 | A1CF | 9603 |
| 3 | 3 | ENSG00000175899 | A2M | 6318 |
| 4 | 4 | ENSG00000245105 | A2M-AS1 | 2948 |
| ... | ... | ... | ... | ... |
| 60659 | 60659 | ENSG00000288719 | RP4-669P10.21 | 4252 |
| 60660 | 60660 | ENSG00000288720 | RP11-852E15.3 | 7007 |
| 60661 | 60661 | ENSG00000288721 | RP5-973N23.5 | 7765 |
| 60662 | 60662 | ENSG00000288723 | RP11-553N16.6 | 1015 |
| 60663 | 60663 | ENSG00000288724 | RP13-546I2.2 | 625 |
60664 rows × 4 columns
You can see the total number of genes represented by the number of rows. This number is actually misleading because it is the join of all genes in the Census. However we know that the lung data comes from a subset of datasets.
So let’s take a look at the number of genes that were measured in each of those datasets.
To accomplish this we can use the “dataset presence matrix” at census["census_data"]["homo_sapiens"].ms["RNA"]["feature_dataset_presence_matrix"]. This is a boolean matrix N x M where N is the number of datasets and M is the number of genes in the Census.
So we can select the rows corresponding to the lung datasets and perform a row-wise sum.
[13]:
presence_matrix = cellxgene_census.get_presence_matrix(census, "Homo sapiens", "RNA")
presence_matrix = presence_matrix[dataset_cell_counts.soma_joinid, :]
[14]:
presence_matrix.sum(axis=1).A1
[14]:
array([17811, 50259, 44150, 34265, 22447, 23642, 26347, 20921, 24672,
27705, 27243, 26323, 27181, 23203, 57042, 32610, 29620, 26454,
23705, 38676, 47307, 23740, 22552, 20594, 19952], dtype=uint64)
[15]:
genes_measured = presence_matrix.sum(axis=1).A1
dataset_cell_counts["genes_measured"] = genes_measured
dataset_cell_counts
[15]:
| count | collection_name | dataset_title | soma_joinid | genes_measured | |
|---|---|---|---|---|---|
| dataset_id | |||||
| 1e6a6ef9-7ec9-4c90-bbfb-2ad3c3165fd1 | 1028006 | High-resolution single-cell atlas reveals dive... | The single-cell lung cancer atlas (LuCA) -- ex... | 314 | 17811 |
| 9f222629-9e39-47d0-b83f-e08d610c7479 | 784630 | The integrated Human Lung Cell Atlas | An integrated cell atlas of the human lung in ... | 56 | 50259 |
| f7c1c579-2dc0-47e2-ba19-8165c5a0e353 | 217738 | A human cell atlas of fetal gene expression | Survey of human embryonic development | 483 | 44150 |
| d8da613f-e681-4c69-b463-e94f5e66847f | 116313 | A molecular single-cell lung atlas of lethal C... | A molecular single-cell lung atlas of lethal C... | 80 | 34265 |
| 576f193c-75d0-4a11-bd25-8676587e6dc2 | 90384 | HTAN MSK - Single cell profiling reveals novel... | Combined samples | 377 | 22447 |
| d41f45c1-1b7b-4573-a998-ac5c5acb1647 | 82991 | HTAN MSK - Transcriptional connectivity of reg... | Transcriptional connectivity of regulatory T c... | 58 | 23642 |
| 3dc61ca1-ce40-46b6-8337-f27260fd9a03 | 71752 | A human fetal lung cell atlas uncovers proxima... | All cells | 325 | 26347 |
| 8c42cfd0-0b0a-46d5-910c-fc833d83c45e | 60993 | A molecular cell atlas of the human lung from ... | Krasnow Lab Human Lung Cell Atlas, 10X | 592 | 20921 |
| 2672b679-8048-4f5e-9786-f1b196ccfd08 | 57019 | scRNA-seq assessment of the human lung, spleen... | Lung Parenchyma | 416 | 24672 |
| 9dbab10c-118d-496b-966a-67f1763a6b7d | 49014 | COVID-19 immune features revealed by a large-s... | Large-scale single-cell analysis reveals criti... | 482 | 27705 |
| 9968be68-ab65-4a38-9e1a-c9b6abece194 | 47909 | Charting human development using a multi-endod... | Developing Human Atlas | 78 | 27243 |
| 3de0ad6d-4378-4f62-b37b-ec0b75a50d94 | 46500 | LungMAP — Human data from a broad age healthy ... | Single-cell multiomic profiling of human lungs... | 456 | 26323 |
| 2f132ec9-24b5-422f-9be0-ccef03b4fe28 | 39778 | SARS-CoV-2 receptor ACE2 and TMPRSS2 are prima... | Lung | 312 | 27181 |
| 1e5bd3b8-6a0e-4959-8d69-cafed30fe814 | 35699 | Emphysema Cell Atlas | immune cells | 130 | 23203 |
| 53d208b0-2cfd-4366-9866-c3c6114081bc | 35682 | Tabula Sapiens | Tabula Sapiens - All Cells | 475 | 57042 |
| 1b9d8702-5af8-4142-85ed-020eb06ec4f6 | 35419 | Cross-tissue immune cell analysis reveals tiss... | Global | 411 | 32610 |
| 4ed927e9-c099-49af-b8ce-a2652d069333 | 35284 | Single-nucleus cross-tissue molecular referenc... | Single-nucleus cross-tissue molecular referenc... | 367 | 29620 |
| 2adb1f8a-a6b1-4909-8ee8-484814e2d4bf | 33698 | Construction of a human cell landscape at sing... | Construction of a human cell landscape at sing... | 590 | 26454 |
| 4b6af54a-4a21-46e0-bc8d-673c0561a836 | 18386 | Emphysema Cell Atlas | non-immune cells | 128 | 23705 |
| 01209dce-3575-4bed-b1df-129f57fbc031 | 11059 | Single-cell transcriptomics of human T cells r... | Single-cell transcriptomics of human T cells r... | 531 | 38676 |
| e04daea4-4412-45b5-989e-76a9be070a89 | 8657 | A molecular cell atlas of the human lung from ... | Krasnow Lab Human Lung Cell Atlas, Smart-seq2 | 591 | 47307 |
| f9846bb4-784d-4582-92c1-3f279e4c6f0c | 176 | A human fetal lung cell atlas uncovers proxima... | Fibroblast and smooth muscle | 317 | 23740 |
| f64e1be1-de15-4d27-8da4-82225cd4c035 | 55 | HTAN MSK - Single cell profiling reveals novel... | Immune cells | 370 | 22552 |
| 810ac45f-8969-4698-b42c-652f802f75c2 | 10 | A human fetal lung cell atlas uncovers proxima... | Endothelium | 320 | 20594 |
| 0ba16f4b-cb87-4fa3-9363-19fc51eec6e7 | 4 | A human fetal lung cell atlas uncovers proxima... | Myeloid | 326 | 19952 |
You can see the genes measured in each dataset represented in genes_measured. Now lets get the genes that were measured in all datasets.
[16]:
var_somaid = np.nonzero(presence_matrix.sum(axis=0).A1 == presence_matrix.shape[0])[0].tolist()
[17]:
lung_var = lung_var.query(f"soma_joinid in {var_somaid}")
lung_var
[17]:
| soma_joinid | feature_id | feature_name | feature_length | |
|---|---|---|---|---|
| 0 | 0 | ENSG00000121410 | A1BG | 3999 |
| 3 | 3 | ENSG00000175899 | A2M | 6318 |
| 8 | 8 | ENSG00000128274 | A4GALT | 3358 |
| 10 | 10 | ENSG00000094914 | AAAS | 4727 |
| 11 | 11 | ENSG00000081760 | AACS | 16039 |
| ... | ... | ... | ... | ... |
| 29951 | 29951 | ENSG00000177272 | KCNA3 | 2476 |
| 30157 | 30157 | ENSG00000184709 | LRRC26 | 1209 |
| 30185 | 30185 | ENSG00000087250 | MT3 | 1679 |
| 30202 | 30202 | ENSG00000136352 | NKX2-1 | 3165 |
| 30512 | 30512 | ENSG00000231439 | WASIR2 | 1054 |
11595 rows × 4 columns
The number of rows represents the genes that were measured in all lung datasets.
Summary of lung metadata
In the previous sections, using the Census we learned the following information:
The total number of unique lung cells and their composition for:
Number of datasets.
Number sequencing technologies, most of which are 10x
Mostly human data, but some diseases exist, primarily “lung adenocarcinoma” and “COVID-19 infected”
No sex biases.
Mostly data from cells (~80%) rather than nucleus (~20%)
A total of ~12k genes were measured across all cells.
Fetching all single-cell human lung data from the Census
Since loading the entire lung data is resource-intensive, for the sake of this exercise let’s load a subset of the lung data into an anndata.AnnData object and perform some exploratory analysis.
We will subset to 100,000 random unique cells using the lung_obs pandas.DataFrame we previously created.
[18]:
lung_cell_subsampled_n = 100000
lung_cell_subsampled_ids = lung_obs["soma_joinid"].sample(lung_cell_subsampled_n, random_state=1).tolist()
Now we can directly use the values of soma_joinid for querying the Census data and obtaining an AnnData object.
[19]:
lung_gene_ids = lung_var["soma_joinid"].to_numpy()
lung_adata = cellxgene_census.get_anndata(
census,
organism="Homo sapiens",
obs_coords=lung_cell_subsampled_ids,
var_coords=lung_gene_ids,
)
lung_adata.var_names = lung_adata.var["feature_name"]
[20]:
lung_adata
[20]:
AnnData object with n_obs × n_vars = 100000 × 11595
obs: 'soma_joinid', 'dataset_id', 'assay', 'assay_ontology_term_id', 'cell_type', 'cell_type_ontology_term_id', 'development_stage', 'development_stage_ontology_term_id', 'disease', 'disease_ontology_term_id', 'donor_id', 'is_primary_data', 'self_reported_ethnicity', 'self_reported_ethnicity_ontology_term_id', 'sex', 'sex_ontology_term_id', 'suspension_type', 'tissue', 'tissue_ontology_term_id', 'tissue_general', 'tissue_general_ontology_term_id'
var: 'soma_joinid', 'feature_id', 'feature_name', 'feature_length'
We are done with the census, so close it
[21]:
census.close()
del census
Calculating QC metrics of the lung data
Now let’s take a look at some QC metrics
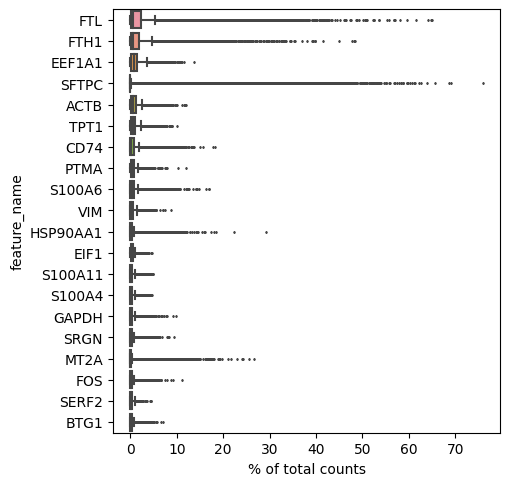
Top genes per cell
[22]:
sc.pl.highest_expr_genes(lung_adata, n_top=20)
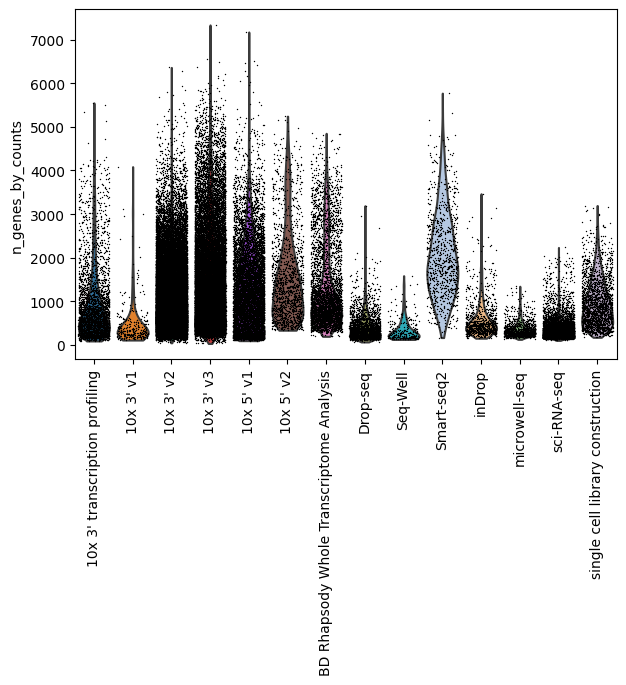
Number of sequenced genes by assay
[23]:
sc.pp.calculate_qc_metrics(lung_adata, percent_top=None, log1p=False, inplace=True)
sc.pl.violin(lung_adata, "n_genes_by_counts", groupby="assay", jitter=0.4, rotation=90)
Total counts by assay
[24]:
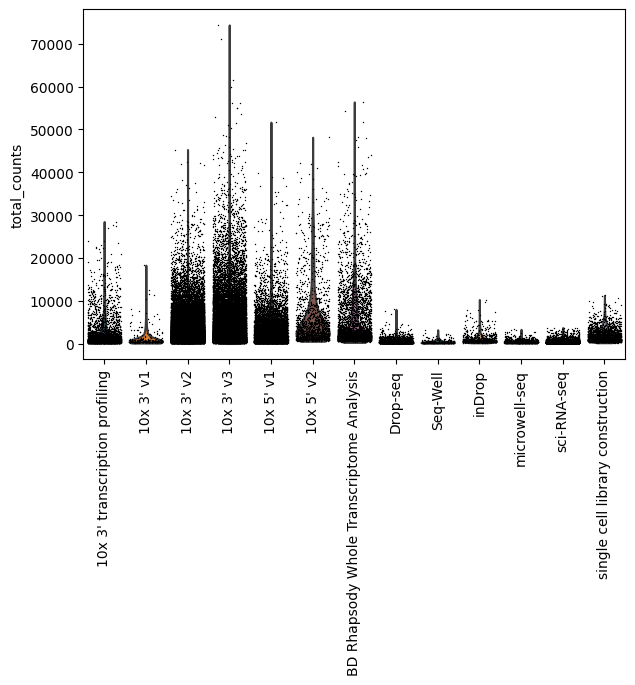
sc.pl.violin(lung_adata, "total_counts", groupby="assay", jitter=0.4, rotation=90)
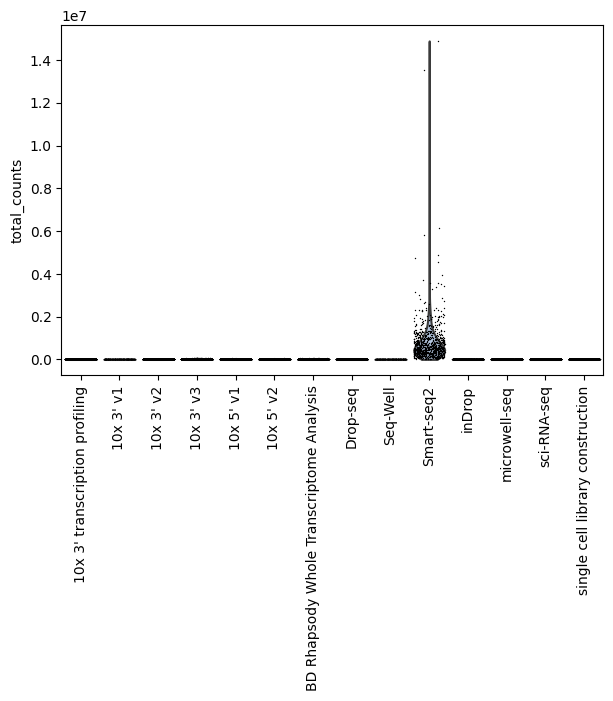
You can see that Smart-Seq2 is an outlier for the total counts per cell, so let’s exlcude it to see how the rest of the assays look like
[25]:
sc.pl.violin(
lung_adata[lung_adata.obs["assay"] != "Smart-seq2",],
"total_counts",
groupby="assay",
jitter=0.4,
rotation=90,
)
Creating a normalized expression layer and embeddings
Let’s perform a bread and butter normalization and take a look at UMAP embeddings, but for all the data below we’ll exclude Smart-seq2 as this requires an extra step to normalize based on gene lengths
[26]:
lung_adata = lung_adata[lung_adata.obs["assay"] != "Smart-seq2",].copy()
lung_adata.layers["counts"] = lung_adata.X
Now let’s do some basic normalization:
Normalize by sequencing depth
Transform to log-scale
Select 500 highly variable genes
Scale values across the gene axis
[27]:
sc.pp.normalize_total(lung_adata, target_sum=1e4)
sc.pp.log1p(lung_adata)
sc.pp.highly_variable_genes(lung_adata, n_top_genes=500, flavor="seurat_v3", layer="counts")
lung_adata = lung_adata[:, lung_adata.var.highly_variable]
sc.pp.scale(lung_adata, max_value=10)
/home/ssm-user/cellxgene-census/venv/lib/python3.10/site-packages/scanpy/preprocessing/_highly_variable_genes.py:62: UserWarning: `flavor='seurat_v3'` expects raw count data, but non-integers were found.
warnings.warn(
/home/ssm-user/cellxgene-census/venv/lib/python3.10/site-packages/scanpy/preprocessing/_simple.py:843: UserWarning: Received a view of an AnnData. Making a copy.
view_to_actual(adata)
And reduce dimensionality by obtaining UMAP embeddings.
[28]:
sc.tl.pca(lung_adata)
sc.pp.neighbors(lung_adata)
sc.tl.umap(lung_adata)
/home/ssm-user/cellxgene-census/venv/lib/python3.10/site-packages/tqdm/auto.py:21: TqdmWarning: IProgress not found. Please update jupyter and ipywidgets. See https://ipywidgets.readthedocs.io/en/stable/user_install.html
from .autonotebook import tqdm as notebook_tqdm
And plot these embeddings.
[29]:
n_cell_types = len(lung_adata.obs["cell_type"].drop_duplicates())
from random import randint
colors = []
for i in range(len(lung_adata.obs["cell_type"].drop_duplicates())):
colors.append("#%06X" % randint(0, 0xFFFFFF))
[30]:
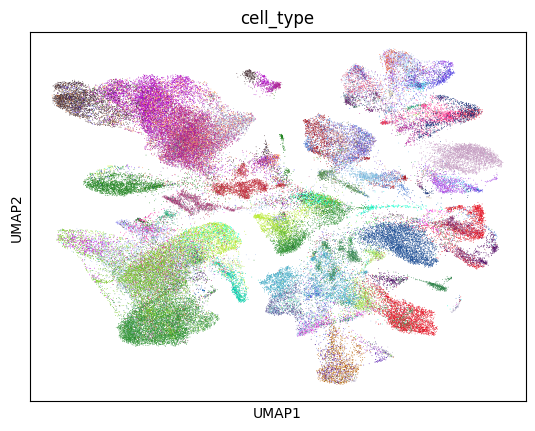
sc.pl.umap(lung_adata, color="cell_type", palette=colors, legend_loc=None)
/home/ssm-user/cellxgene-census/venv/lib/python3.10/site-packages/scanpy/plotting/_tools/scatterplots.py:392: UserWarning: No data for colormapping provided via 'c'. Parameters 'cmap' will be ignored
cax = scatter(
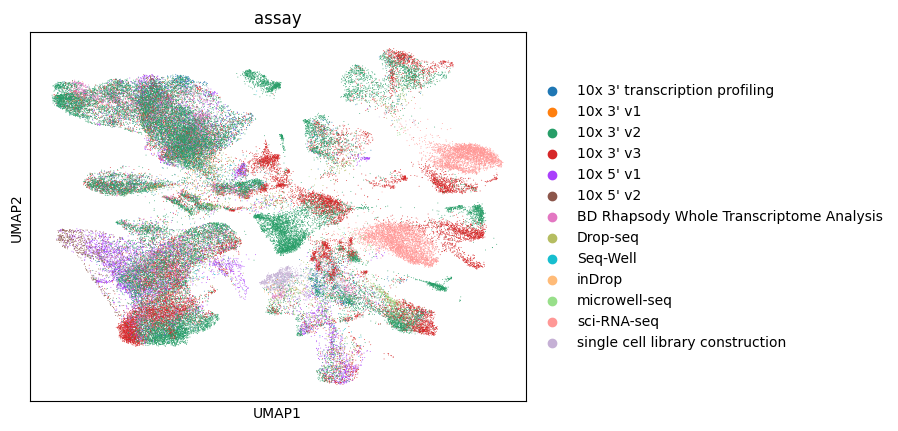
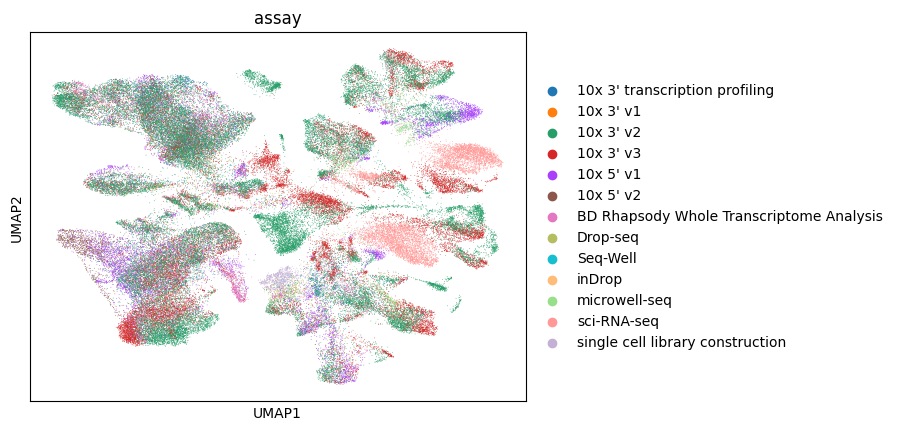
Let’s color by assay.
[31]:
sc.pl.umap(lung_adata, color="assay")
/home/ssm-user/cellxgene-census/venv/lib/python3.10/site-packages/scanpy/plotting/_tools/scatterplots.py:392: UserWarning: No data for colormapping provided via 'c'. Parameters 'cmap' will be ignored
cax = scatter(
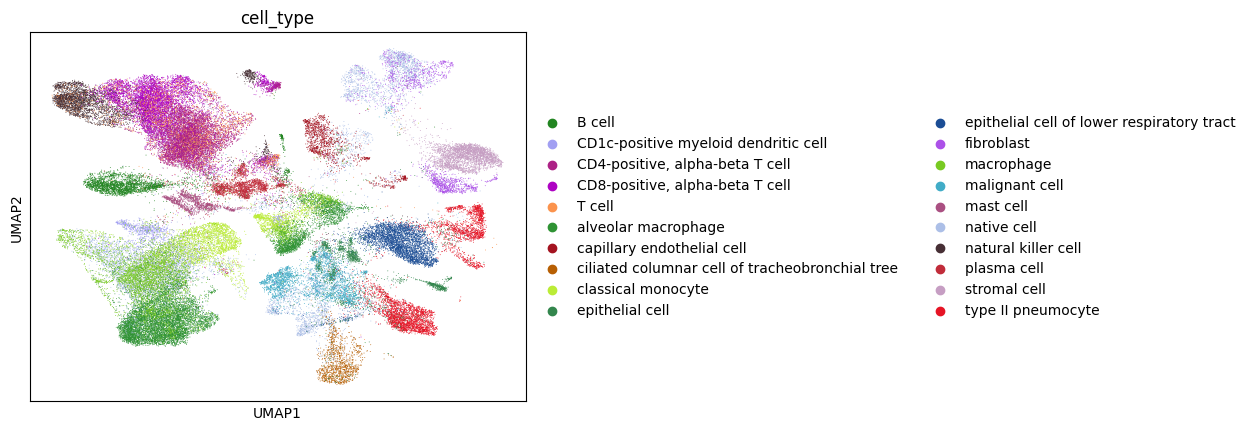
Given the high number of cell types it makes it hard to visualize, so let’s look at the top 20 most abundant cell types.
[32]:
top_cell_types = lung_adata.obs["cell_type"].value_counts()
top_cell_types = list(top_cell_types.reset_index().head(20)["cell_type"])
[33]:
lung_adata_top_cell_types = lung_adata[[i in top_cell_types for i in lung_adata.obs["cell_type"]], :]
sc.pl.umap(lung_adata_top_cell_types, color="cell_type")
/home/ssm-user/cellxgene-census/venv/lib/python3.10/site-packages/scanpy/plotting/_tools/scatterplots.py:392: UserWarning: No data for colormapping provided via 'c'. Parameters 'cmap' will be ignored
cax = scatter(
Let’s color by assay of this subset of the data.
[34]:
sc.pl.umap(lung_adata_top_cell_types, color="assay")
/home/ssm-user/cellxgene-census/venv/lib/python3.10/site-packages/scanpy/plotting/_tools/scatterplots.py:392: UserWarning: No data for colormapping provided via 'c'. Parameters 'cmap' will be ignored
cax = scatter(